Frequently Asked Questions
Have a question not answered below? Get in touch.1. What is calcifediol?
Your Subtitle Goes Here
Figure 1: Images of Colecalciferol, Calcifediol and Calcitriol
2. In what ways do the contrasting pharmacokinetics of calcifediol alter its characteristics in comparison to colecalciferol?
Your Subtitle Goes Here
- Jodar E, Campusano C, de Jongh RT, Holick MF. Calcifediol: a review of its pharmacological characteristics and clinical use in correcting vitamin D deficiency. Eur J Nutr. 2023;62(4):1579-1597. doi:10.1007/s00394-023-03103-1.
Treatment
3. What do the 25(OH)D levels tell us about the patient’s vitamin D level?
Your Subtitle Goes Here
The 25(OH)D (also known as 25-hydroxyvitamin D) levels are a measure of concentration of 25-hydroxyvitamin D in the bloodstream. It is the major circulating form of vitamin D and serves as reliable indicator of an individual’s vitamin D status. Hence, measuring 25(OH)D is commonly used to assess an individual’s vitamin D status.2
| 25(OH)D concentration | Classification |
| <25 nmol/L | Deficient |
| 25-50 nmol/L | Insufficient |
| >50 nmol/L | Optimal |
Table 1: Classification of vitamin D status by 25(OH)D concentration* 3
*Please consider that optimal range for 25(OH)D levels may vary based on individual factors, such as age, health conditions, and specific medical recommendations.
References
2. Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv Nutr. 2017;8(6):947-957. doi:10.3945/an.117.015578
3. Vitamin D deficiency in adults | Health topics A to Z | CKS | NICE. Accessed June 9, 2023. https://cks.nice.org.uk/topics/vitamin-d-deficiency-in-adults/
4. What is the recommended frequency for monitoring patients on maintenance treatment?
Your Subtitle Goes Here
5. Do I need to worry about adherence, compliance and polypharmacy when prescribing Domnisol (calcifediol monohydrate)?
Your Subtitle Goes Here
Figure 2: Patient preference for different dosing frequency of vitamin D supplements6
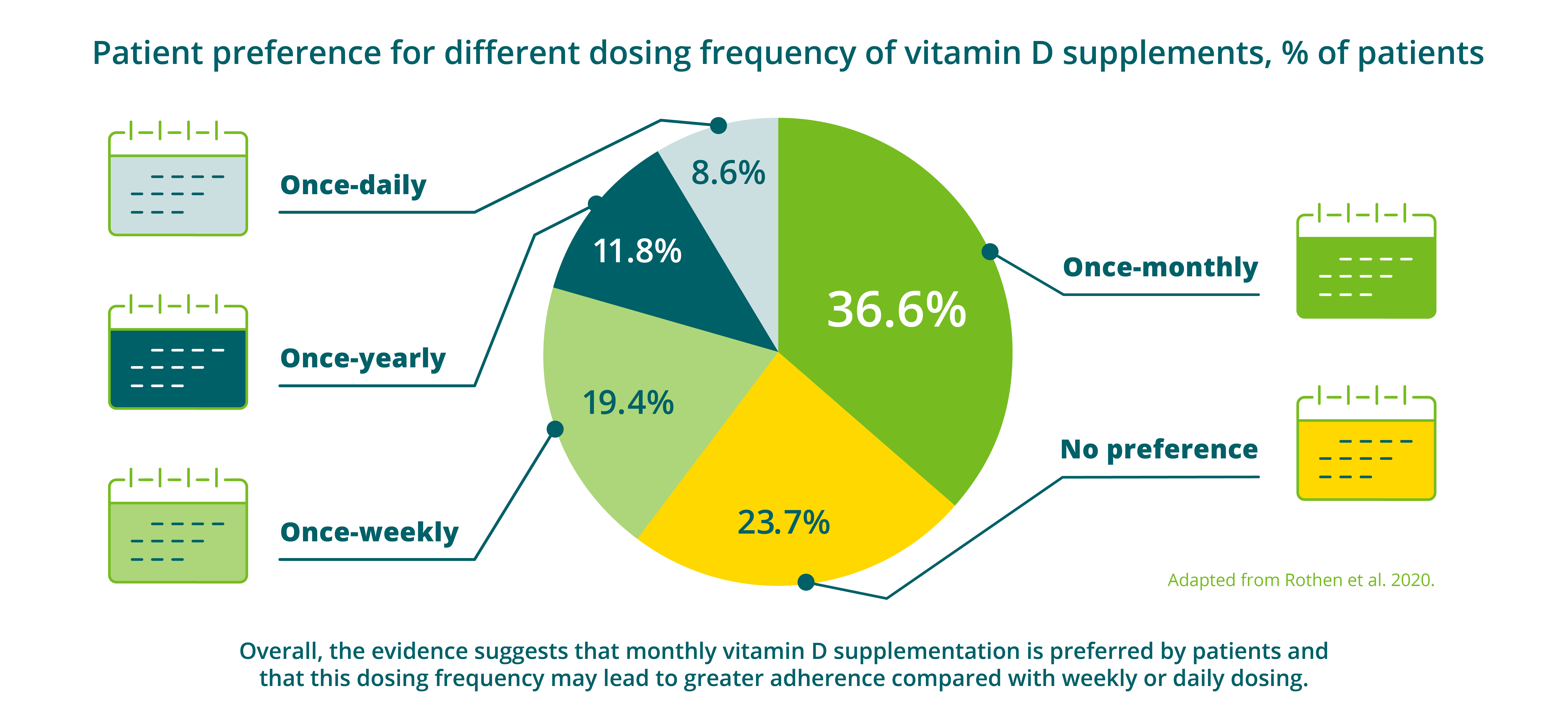 References
4. Barnett NL, Oboh L, Smith K. Patient-centred management of polypharmacy: a process for practice. Eur J Hosp Pharm. 2016;23(2):113-117. doi:10.1136/ejhpharm-2015-000762
5. Kupisz-Urbańska M, Płudowski P, Marcinowska-Suchowierska E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients. 2021;13(4):1247. doi:10.3390/nu13041247
6. Rothen JP, Rutishauser J, Walter PN, Hersberger KE, Arnet I. Oral intermittent vitamin D substitution: influence of pharmaceutical form and dosage frequency on medication adherence: a randomized clinical trial. BMC Pharmacol Toxicol. 2020;21:51. doi:10.1186/s40360-020-00430-5
References
4. Barnett NL, Oboh L, Smith K. Patient-centred management of polypharmacy: a process for practice. Eur J Hosp Pharm. 2016;23(2):113-117. doi:10.1136/ejhpharm-2015-000762
5. Kupisz-Urbańska M, Płudowski P, Marcinowska-Suchowierska E. Vitamin D Deficiency in Older Patients-Problems of Sarcopenia, Drug Interactions, Management in Deficiency. Nutrients. 2021;13(4):1247. doi:10.3390/nu13041247
6. Rothen JP, Rutishauser J, Walter PN, Hersberger KE, Arnet I. Oral intermittent vitamin D substitution: influence of pharmaceutical form and dosage frequency on medication adherence: a randomized clinical trial. BMC Pharmacol Toxicol. 2020;21:51. doi:10.1186/s40360-020-00430-56. Are there any benefits of continuous treatment after achieving required vitamin D levels?
Your Subtitle Goes Here
Figure 3: Mean serum 25(OH)D concentrations during study (PP population, n = 170) 7
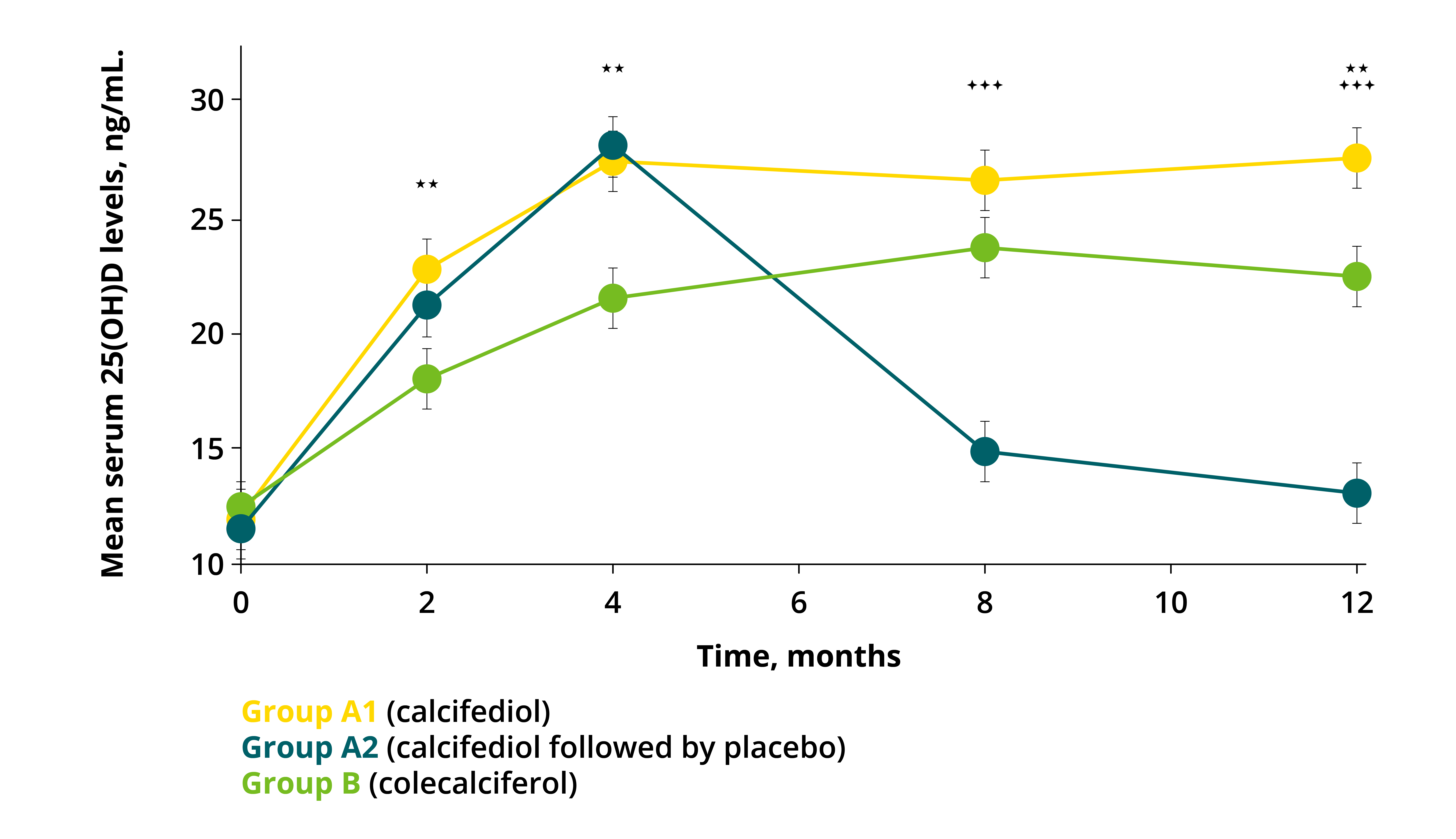 A strong decrease in 25(OH)D levels to baseline can be observed 4 months after calcifediol withdrawal (Group A2) compared with sustained administration (Group A1). Statistical comparisons are for Group A1 versus Group A2 ( p < 0.05; p < 0.001; +++, p < 0.0001) and for Group A1 versus Group B (*, p < 0.05; **, p < 0.001; p < 0.0001). Error bars: 95% CI.7
References
7. Pérez-Castrillón JL, Dueñas-Laita A, Gómez-Alonso C, et al. Long-Term Treatment and Effect of Discontinuation of Calcifediol in Postmenopausal Women with Vitamin D Deficiency: A Randomized Trial. J Bone Miner Res. 2023;38(4):471-479. doi:10.1002/jbmr.4776
A strong decrease in 25(OH)D levels to baseline can be observed 4 months after calcifediol withdrawal (Group A2) compared with sustained administration (Group A1). Statistical comparisons are for Group A1 versus Group A2 ( p < 0.05; p < 0.001; +++, p < 0.0001) and for Group A1 versus Group B (*, p < 0.05; **, p < 0.001; p < 0.0001). Error bars: 95% CI.7
References
7. Pérez-Castrillón JL, Dueñas-Laita A, Gómez-Alonso C, et al. Long-Term Treatment and Effect of Discontinuation of Calcifediol in Postmenopausal Women with Vitamin D Deficiency: A Randomized Trial. J Bone Miner Res. 2023;38(4):471-479. doi:10.1002/jbmr.47767. What unit of measurement is used to determine the dosages for Domnisol (calcifediol monohydrate)?
Your Subtitle Goes Here
Patient Groups
8. Is Domnisol (calcifediol monohydrate) suitable for use in obese patients?
Your Subtitle Goes Here
9. Is Domnisol (calcifediol monohydrate) suitable for use in patients suffering with advanced liver disease?
Your Subtitle Goes Here
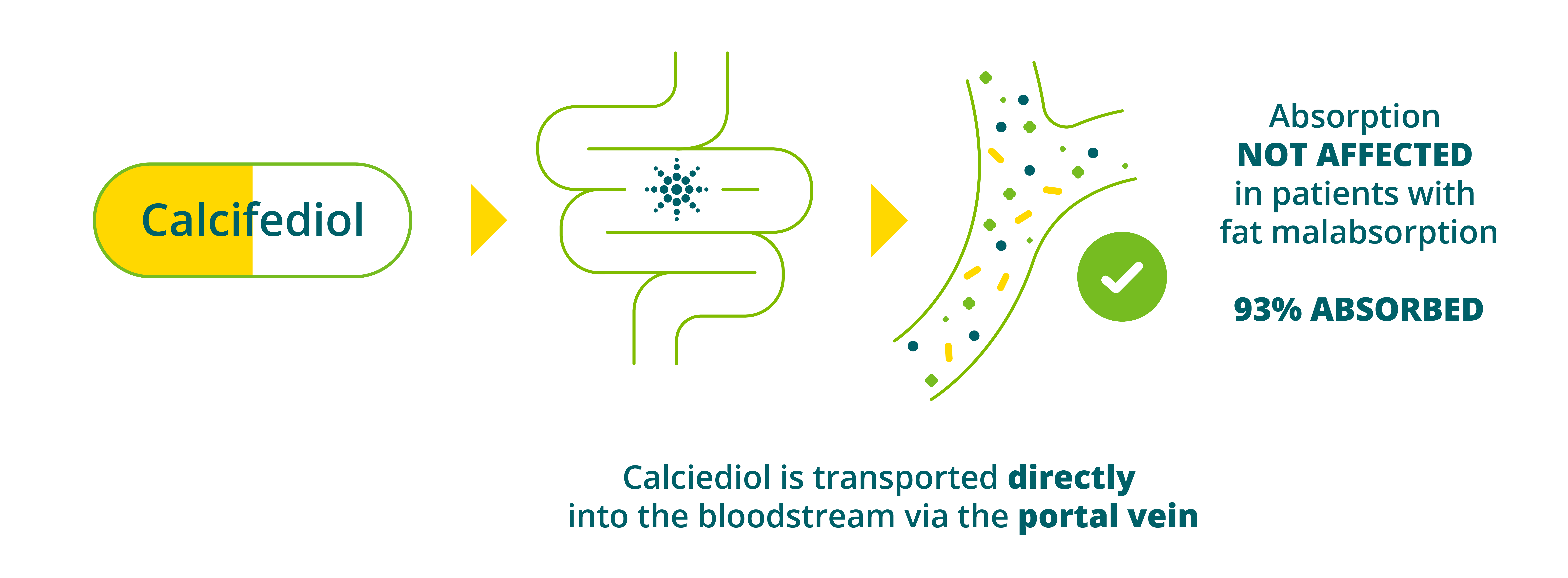
10. Is Domnisol (calcifediol monohydrate) suitable in patients with decreased intestinal absorption?
Your Subtitle Goes Here
Figure 5: Calcifediol being transported directly into the bloodstream via the portal vein9
 References
9. Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697-1711. doi:10.1007/s00198-018-4520-y
References
9. Quesada-Gomez JM, Bouillon R. Is calcifediol better than cholecalciferol for vitamin D supplementation? Osteoporos Int. 2018;29(8):1697-1711. doi:10.1007/s00198-018-4520-yIngredients and Other Questions
11. What is the source of gelatine used?
Your Subtitle Goes Here
Domnisol (calcifediol monohydrate) contains gelatine that is from bovine source.
12. Does Domnisol (calcifediol monohydrate) contain lactose?
Your Subtitle Goes Here
Domnisol (calcifediol monohydrate) does not contain lactose.

