In June 2025, the indication for Slenyto was extended to include:
- Treatment of insomnia in children and adolescents aged 2-18 years with Autism Spectrum Disorder (ASD), and / or neurogenetic disorders with aberrant diurnal melatonin secretion and /or nocturnal awakenings, where sleep hygiene measures have been insufficient.
- Treatment of insomnia in children and adolescents aged 6-17 years with attention-deficit hyperactivity disorder (ADHD) where sleep hygiene measures have been insufficient.
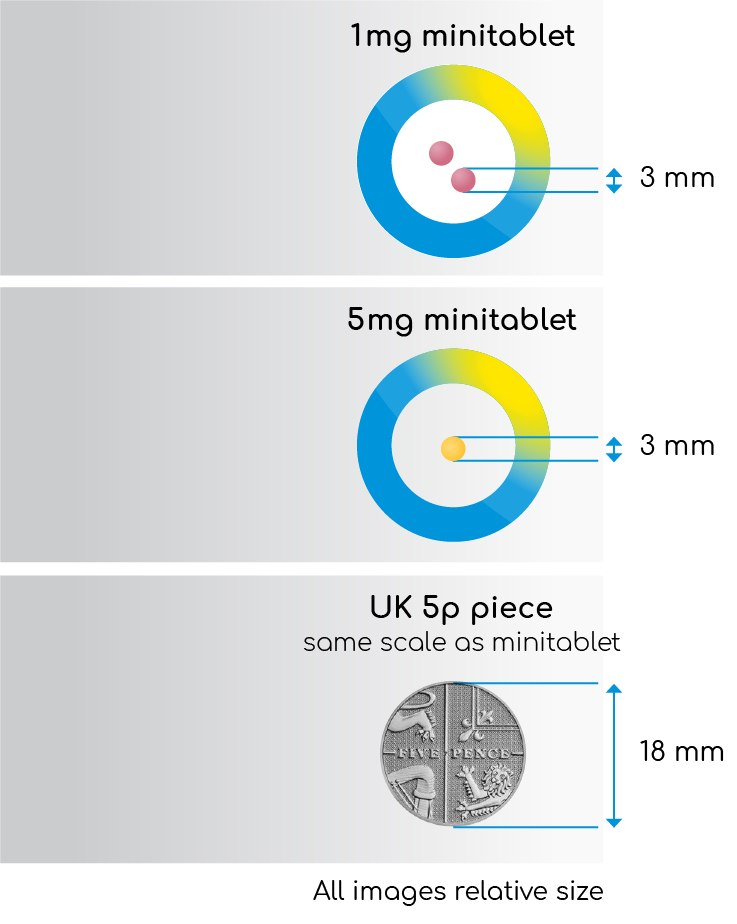
Figure 3. Slenyto (Paediatric Prolonged-Release
Melatonin Tablets) 1mg and 5mg

Slenyto has a Paediatric-Use Marketing Authorisation (PUMA) and is formulated to be both condition (ASD) and age (2-18 years) appropriate. Slenyto mini-tablets (1mg and 5mg) are 3mm in diameter (see Figure 3 - not actual size), flavourless and odourless and the strengths are differentiated by colour. The Summary of Product Characteristics provides further information on co-administration with foodstuffs to facilitate swallowing and improve compliance.1