The Paediatric Investigational Plan (PIP) included a Phase III study demonstrating the product’s short, and long-term efficacy and safety.
The study, in patients who had not previously responded to behavioural intervention, comprised a 2-week, single-blind placebo run-in, followed by a randomised double-blind study of 13 weeks active or placebo treatment, and a further open-label extension comprising 13 weeks of active treatment at final double-blind phase dose (or placebo equivalent dose), and a further 78 weeks treatment with an optional dose escalation to 10mg, and concluded with a 2-week single-blind placebo period (to examine withdrawal effects).3
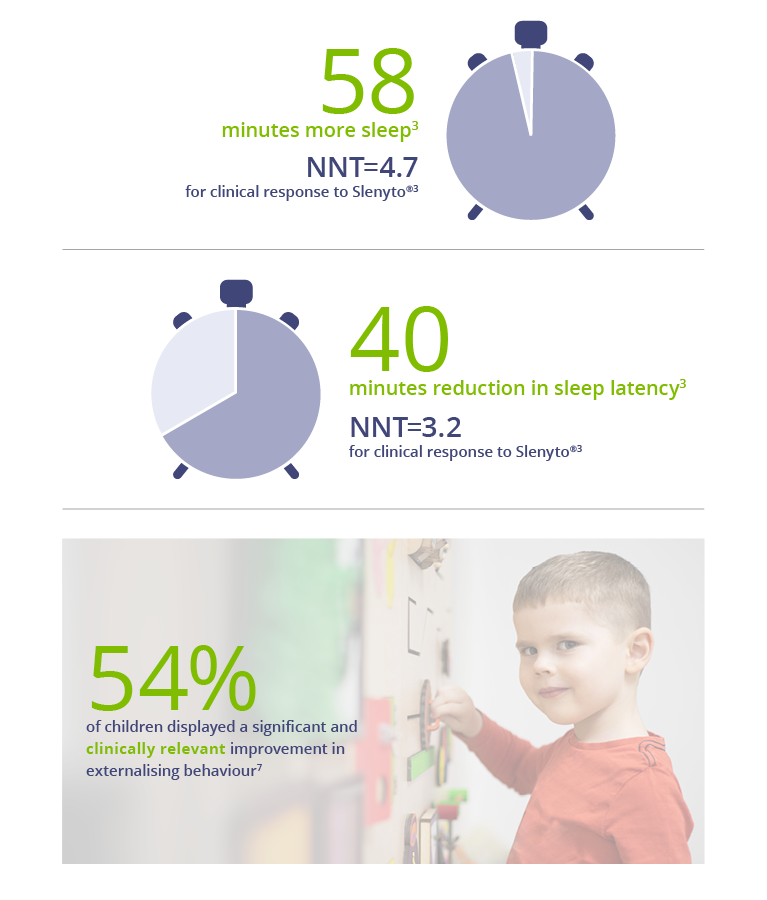
The study provided evidence of significant improvements, over baseline, in total sleep time, sleep initiation (latency) and maintenance, child behaviours (externalising), caregivers’ quality of life and resolution of their own sleep disturbance.
Slenyto demonstrated clinically meaningful* improvement in Total Sleep Time. When used for an initial period of 13 weeks, children treated with Slenyto slept an average of 58 minutes longer per night and fell asleep on average 40 minutes earlier.3,6 Importantly, children treated with Slenyto experienced clinically relevant improvement in externalising behaviour (hyperactivity/inattention and conduct), which correlated well with improvement in caregivers' quality of life.7
Importantly diagnosis (ASD with/ without ADHD or SMS) and co-medication (e.g., stimulants) did not affect Slenyto efficacy outcomes.
The impact of sleep disruption on behaviour can be explained, in part, by the improvement in duration of uninterrupted sleep rather than the improvement in sleep latency. This is of note because the prolonged release formulation of Slenyto, which releases melatonin throughout the night, appears to be effective in improving both sleep onset and sleep maintenance whereas immediate release melatonin formulations are reported as effective in sleep induction but not, or less so, with sleep maintenance.
It is pertinent to ask whether the improvement in parents’ quality of life, subsequent to the improvement in a child’s sleep, might provide a “positive bias” on the parent-completed rating scales of patient behaviour or influence parenting and, therefore, behaviour of children. Yet, the change in caregivers’ quality of life seems strongly related to the improvements in daytime behaviour rather than to the improvements in child’s sleep.
* Clinically meaningful improvement = increase in Total Sleep Time (TST) ≥ 45 mins versus baseline
After 13 weeks treatment with Slenyto...
TWICE as many children met National Sleep Foundation recommendations on Total Sleep Time when treated with Slenyto14,15
Of note, whilst caregivers’ own sleep had not improved significantly at 3 weeks in the Slenyto-treated group (improving only months later), quality of life had improved significantly compared to placebo controls. Moreover at 3 weeks, hyperactivity/inattention scores were significantly improved in Slenyto-treated children.6 These findings could be interpreted as improvement of wellbeing of parents mediated by improvement of daily behaviour in the child and not directly by sleep improvement in the child or parent.
It is, therefore, most likely that the improvement in sleep in the subjects led to the improvement in behaviour and that this improvement is more valuable to the caregivers’ quality of life than the improvement in child sleep per se.7
References:
3. Gringras, P. et al. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948-957
6. Maras, A. et al. Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. Jnl Child and Adolesc Psychpharmacol. 2018; doi 10.1089:1-12
7. Schroder, C. et al. Pediatric Prolonged-Release Melatonin for Sleep in Children with Autism Spectrum Disorder: Impact on Child Behaviour and Care Giver’s Quality of Life. J Autism and Developmental Disorders. 2019
14. Data on File (RAD Neurim Pharmaceuticals EEC Ltd.)
15. National Sleep Foundation https://thensf.org/how-many-hours-of-sleep-do-you-really-need/. (Accessed October 2021)

