In September 2018, Slenyto 1mg and 5mg Tablet (mini-tablets), an age and condition-appropriate paediatric formulation of prolonged-release melatonin for the treatment of insomnia in children with Autistic Spectrum Disorder and/or Smith-Magenis Syndrome, were granted Marketing Authorisations (MAs).
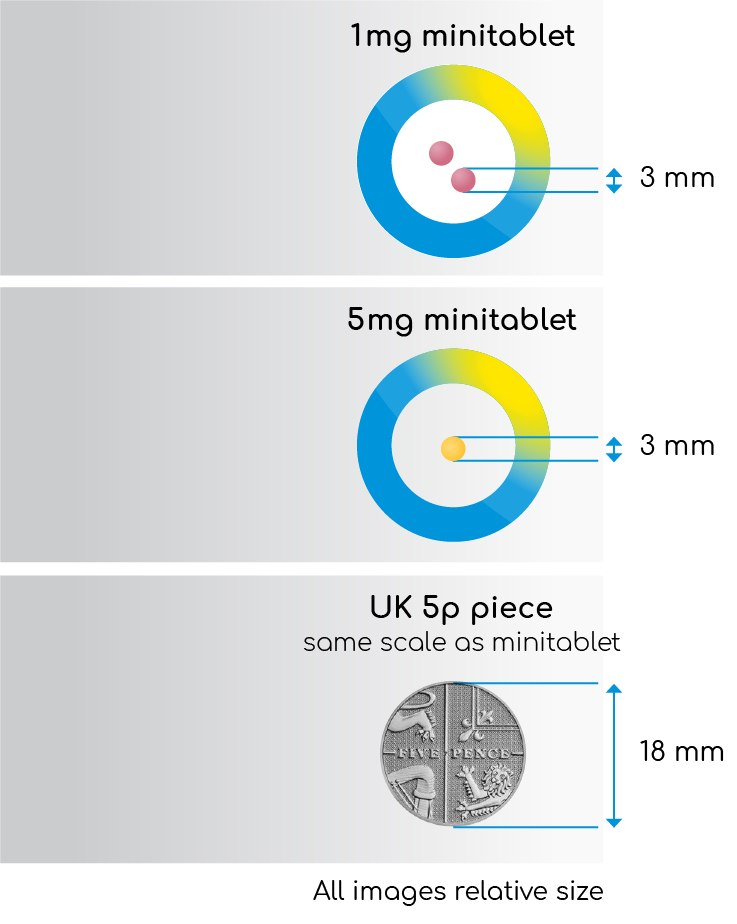
Circadin® (prolonged-release melatonin 2mg indicated for treatment of primary insomnia in patients >55) is not an appropriate product for this patient population. Slenyto has a Paediatric-Use Marketing Authorisation (PUMA) and is formulated to be both condition (ASD) and age (2-18 years) appropriate. Slenyto mini-tablets (1mg and 5mg) are 3mm in diameter (see Figure 3 - not actual size), flavourless and odourless and the strengths are differentiated by colour. The Summary of Product Characteristics provides further information on co-administration with foodstuffs to facilitate swallowing and improve compliance.13
Figure 3. Slenyto (Paediatric Prolonged-Release
Melatonin Tablets) 1mg and 5mg

References:
13. Slenyto® SmPC Accessed November 2021