Insomnia is a widespread problem with a prevalence of 50-80% in children with ASD1
- Limited response to sleep hygiene and/or behavioural interventions2
- Until now, no pharmacological products have been licensed for use in children with insomnia2
- Sleep disturbances exacerbate cognitive performance deficits and behavioural problems in children3
- Parents of children with sleep problems exhibit a higher level of stress than those parents whose children experience no sleep problems4
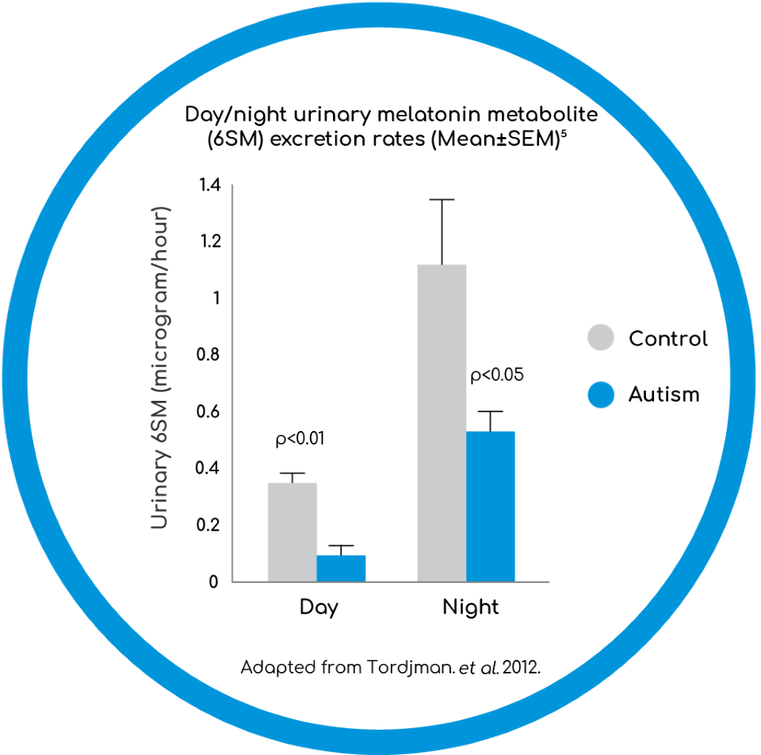
- Abnormal melatonin secretion in children with ASD may explain disturbances in sleep-wake cycle and insomnia3

SEM - standard error mean
Recommendations and current practice
* An A - rating indicates the recommendation is supported by the highest quality evidence.1

Current pharmacological practices for the management of insomnia in children with ASD
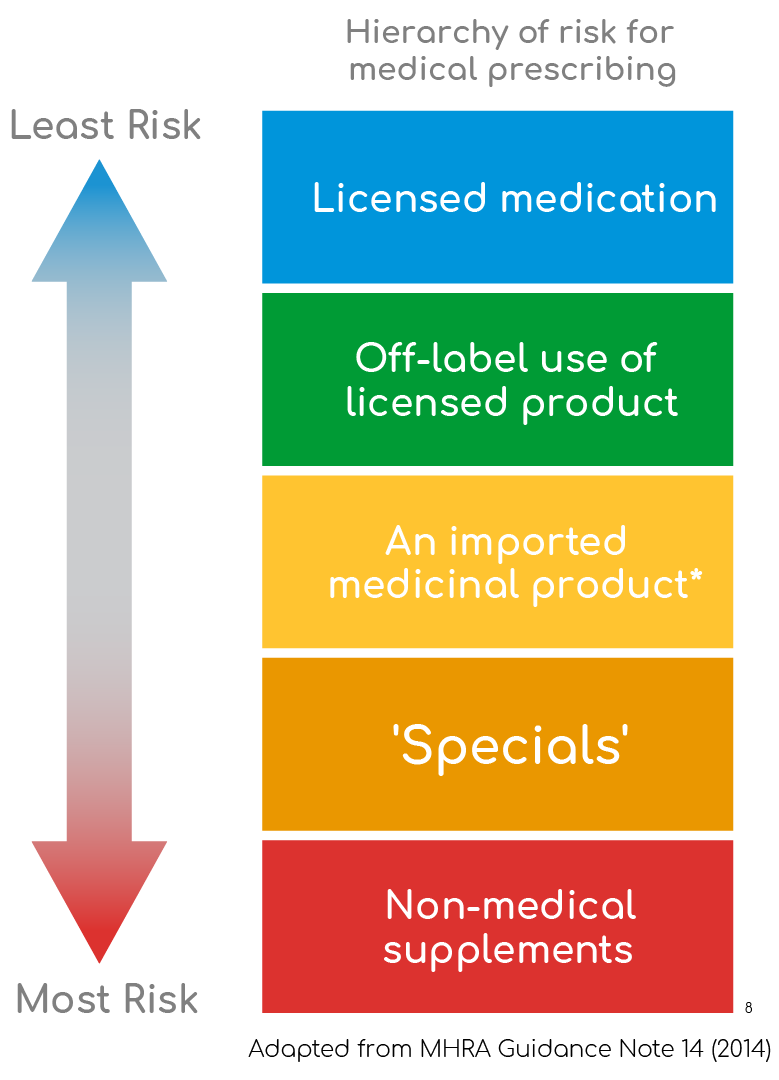
- Unlicensed medication often represents current practice, where previously no licensed alternative existed
- Before prescribing an unlicensed medication a prescriber should be satisfied that:
- An alternative, licensed medicine would not meet the patient’s needs
- Such use would better serve the patient’s needs than an appropriately licensed alternative8
Tactile sensitivities challenge the effective use of non-specialised medications

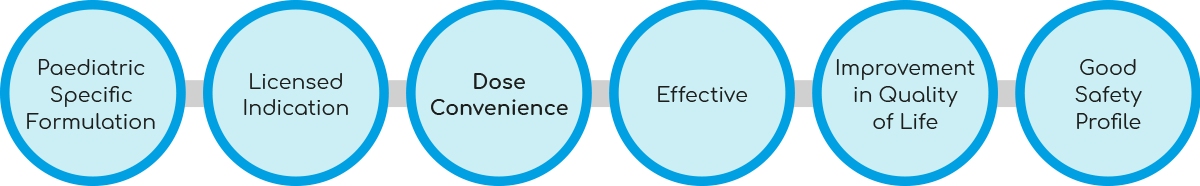
What does an ‘ideal’ pharmacological intervention for insomnia in paediatric ASD look like?
Introducing Slenyto® (prolonged-release melatonin minitablets)
- Prolonged-release, designed to mimic the endogenous production of melatonin
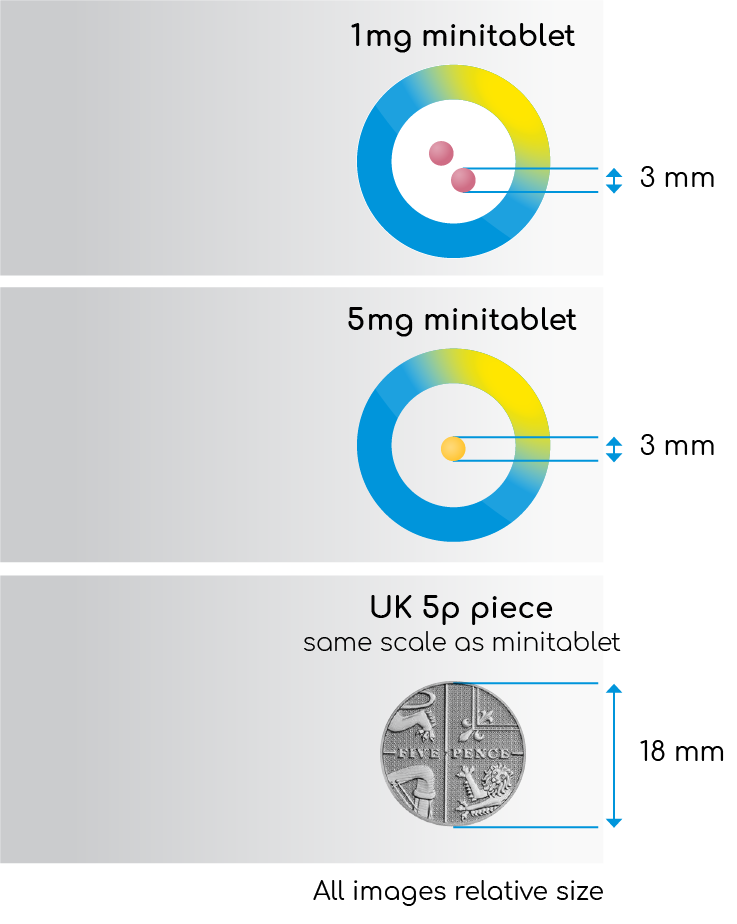
- A paediatric-appropriate, 3 mm-diameter prolonged-release melatonin minitablet12
- Flavourless and odourless
- Easy to swallow, with or without water
- No need to crush
- Dose Convenience
- 1mg and 5mg minitablets12
- Once-nightly dose12
- Specifically developed for use in children
- Paediatric-Use Marketing Authorisation (PUMA) approved13
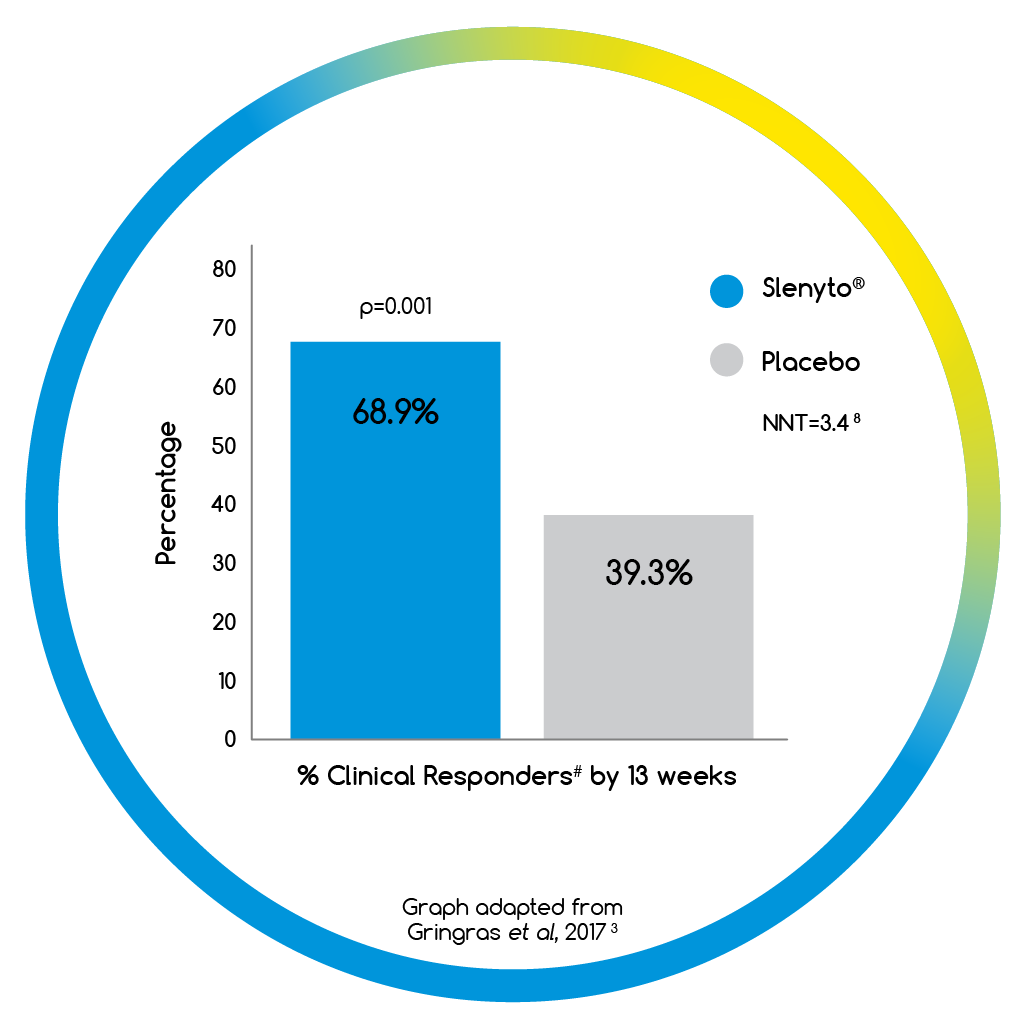
- When treated with Slenyto, 69% of children had a clinically meaningful# response3
- Improvements in Total Sleep Time (TST) and Sleep Latency (SL) did not result in earlier awakening3
- Longest Uninterrupted Sleep Episode (LSE) increased by 78 mins3

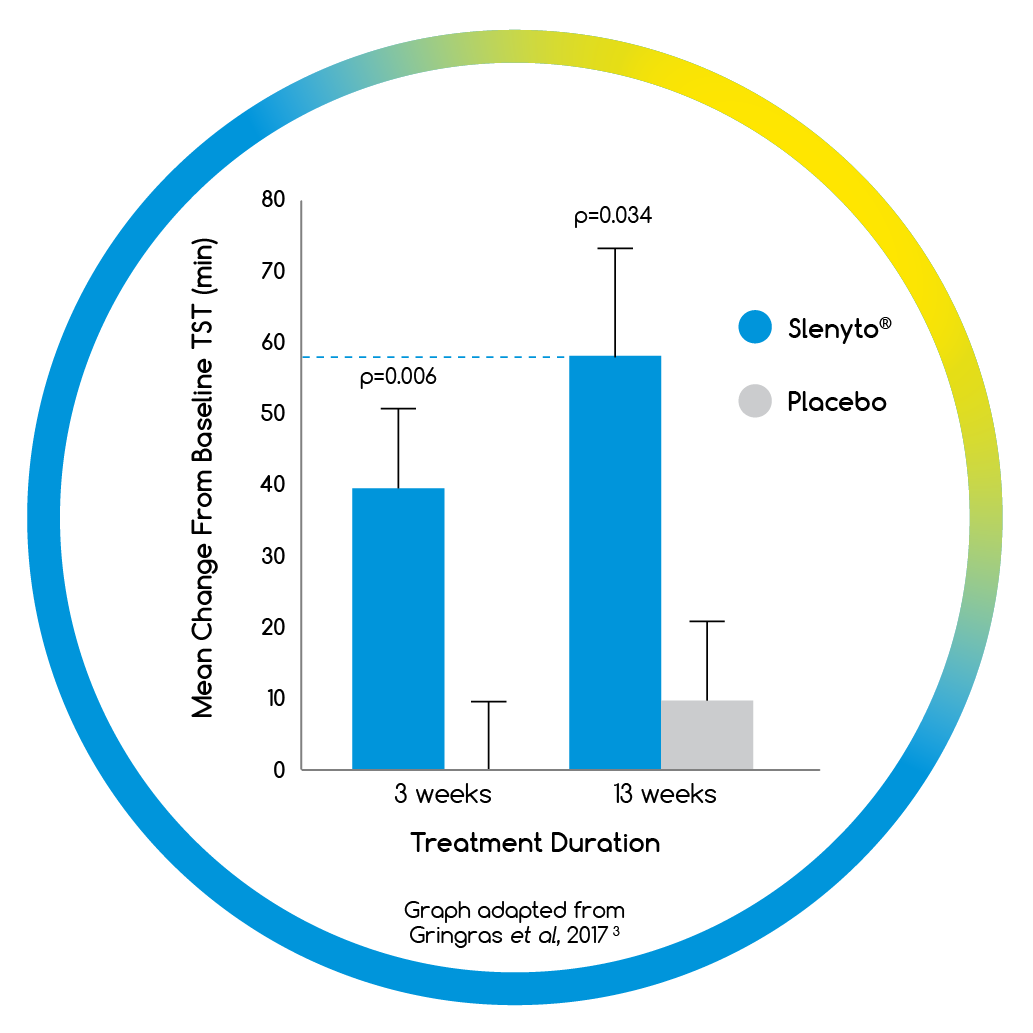
- When used for an initial period of 13 weeks, Slenyto treated children slept an average of 58 minutes longer per night3
- NNT = 4.7 for clinical response# to
Slenyto3

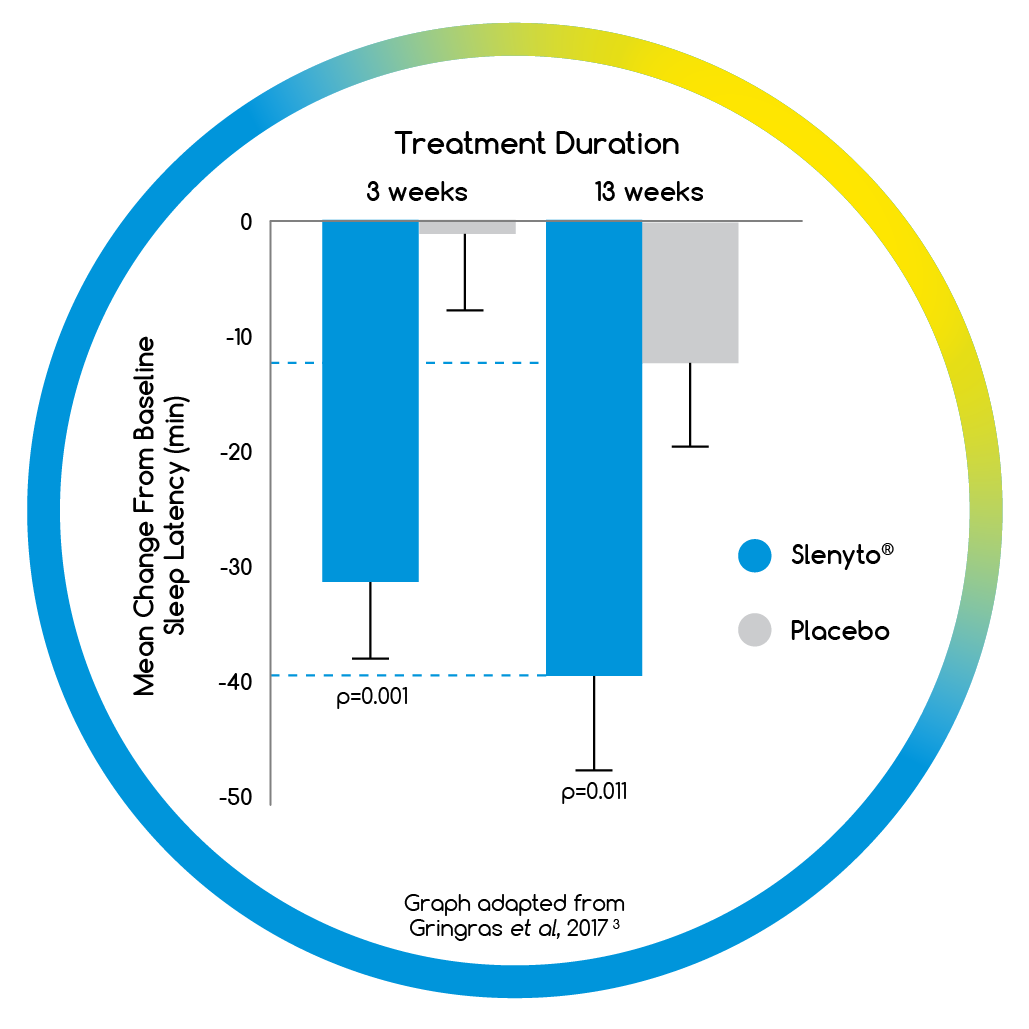
- When used for an initial period of 13 weeks, Slenyto treated children fell asleep on average 40 minutes earlier3
- NNT = 3.2 for clinical response# to Slenyto3
Effective management of sleep problems with Slenyto improves next day behaviour in children with ASD
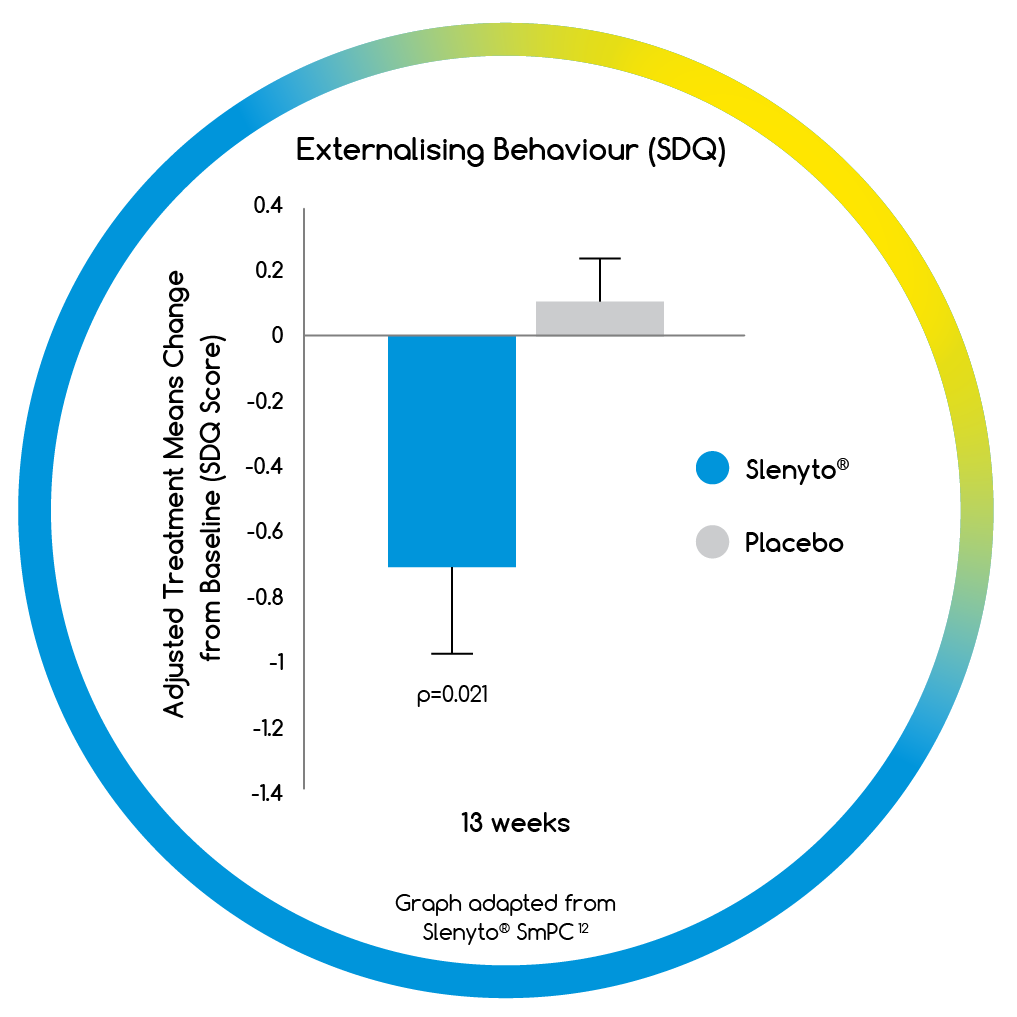
- Treatment with Slenyto resulted in a significant improvement in externalising behaviours (hyperactivity/inattention and conduct scores)12

After 52 weeks of continuous treatment:
- Slenyto treated children:
- Slept on average 62 minutes longer (p=0.007*)2
- Went to sleep on average 49 minutes earlier (p<0.001*) without earlier awakening2
- Experienced an increase in the Longest Uninterrupted Sleep Episode by 89 minutes (p=0.001*)2
- Experienced a reduced number of night-time awakenings by 53% (p=0.001*)2
- 76% of patients responded~ to Slenyto2
- 29% of patients who started treatment on 2mg/day of Slenyto were still effectively managed on this dose2
- Average dose was 5.3mg/day2
* p-values measure change from baseline
~ Responder = overall improvement ≥1 hour in TST, SL or both over baseline, and did not require dose escalation
What does an ‘ideal’ pharmacological intervention for insomnia in paediatric ASD look like?
Effective management of sleep problems in children with ASD can improve the overall health of the family
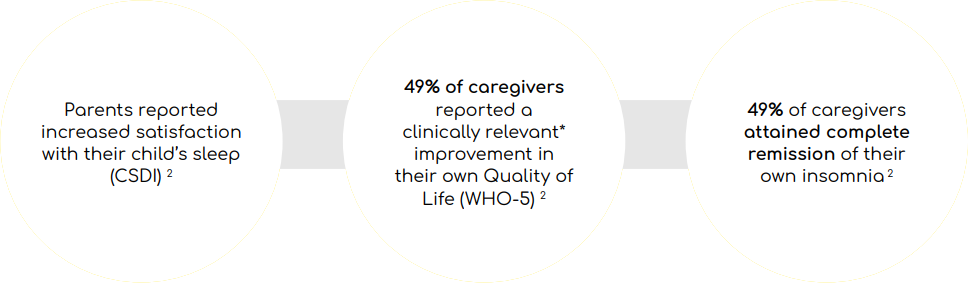
*A change of 10% in WHO-5 score is considered clinically relevant

- Slenyto has been rigorously tested to ensure it has an acceptable safety profile
- PUMA (Paediatric-Use Marketing Authorisation) approved
- There are no Very Common (≥1/10) adverse reactions with Slenyto12
- Common adverse reactions: somnolence, fatigue, mood swings, headache, irritability, aggression, hangover, sinusitis and sudden onset of sleep
- Small increases in BMI and Z-scores were noted, in line with that expected for a developing child over the study time period2
- Slenyto is indicated for the treatment of insomnia in children and adolescents aged 2-18 years with Autism Spectrum Disorder (ASD) and/or Smith-Magenis Syndrome (SMS), where sleep hygiene measures have been insufficient12
- The recommended starting dose is 2mg of Slenyto12
- If required, the dose may be increased to 5mg and further to a maximum dose of 10mg/day12
- Slenyto should be taken once daily, 30 minutes to 1 hour before bedtime, with or after food12
- The minitablet should not be chewed, crushed or broken as it will lose its prolonged-release properties
- Slenyto can be added to food such as yoghurt, orange juice or ice cream to facilitate swallowing
- If mixed with food or drink, the dose should be taken immediately, and the mixture not stored


# Clinically meaningful improvement = increase in TST ≥ 45 mins versus baseline and/or reduction in SL ≥ 15 mins versus baseline
~ Responder = overall improvement ≥ 1 hour in TST, SL or both over baseline, and did not require dose escalation
- By 13 weeks over two thirds of children had a clinically meaningful# improvement in their sleep when using Slenyto2
- Improvements in Total Sleep Time (TST) and Sleep
Latency (SL) did not result in earlier wakening2
- Improvements in Total Sleep Time (TST) and Sleep
- At 52 weeks 76% of children achieved a clinically meaningful~ response to 2, 5 or 10mg of Slenyto3
- The Longest Uninterrupted Sleep Episode (LSE) increased by almost 90 minutes and the Number of Awakenings reduced by 53%3
- Slenyto improved child behaviour and quality of life (QoL) for the family3,12
- Nearly half of caregivers reported a clinically meaningful improvement in their QoL and complete resolution of their own insomnia3
References
1. Howes et al. Autism Spectrum Disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J Psychopharmacol. 2018, January;32(1): 3–29. doi:10.1177/0269881117741766
2. Maras A, et al. Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. Jnl Child and Adolesc Psychpharmacol 2018, DOI: 10.1089/cap.2018.0020
3. Gringras, P. et al. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children With Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2017;56(11):948–957
4. Doo S, Wing YK. Sleep problems of children with pervasive developmental disorders: correlation with parental stress. Dev Med Child Neurol. 2006;48:650-655
5. Tordjman S. et al. Day and night time excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology. 2012;37:1990-7
6. Melke, J. et al. Abnormal melatonin synthesis in autism spectrum disorders. Molecular Psychiatry. 2008, 13, 90-98.
7. Richdale, A.L. and Schreck, K.A. Sleep problems in autism spectrum disorders: Prevalence, nature, & possible biopsychosocial aetiologies. Sleep Medicine Reviews. 2009, 13, 403–411
8. Medicines and Healthcare products Regulatory Agency. The supply of unlicensed medicinal products (“specials”). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/373505/The_supply_of_unlicensed_medicinal_products__specials_.pdf Accessed 22.02.19
9. European Medicines Agency, Guideline on pharmaceutical development of medicines for paediatric use. Available at https://www.ema.europa.eu/en/pharmaceutical-development-medicines-paediatric-use Accessed 11.01.19
10. Gazely, H. Dysphagia for people with Autism and Learning Disabilities. Available at https://www.optionsautism.co.uk/wp-content/uploads/2017/11/Options-Dysphagia-Help-Sheet-Issue-11.pdf Accessed 11.01.19
11. National Institute for Health and Care Excellence, Autism spectrum disorder in under 19s: recognition, referral and diagnosis. CG128, 2011. Available at: https://www.nice.org.uk/guidance/cg128/resources/autism-spectrum-disorder-in-under-19s-recognition-referral-and-diagnosispdf-35109456621253 Accessed 23.01.19
12. Slenyto® SmPC Accessed November 2021